Cataract is the most common cause of avoidable child blindness worldwide. 42T developed a low-cost, handheld device designed for clinicians to use as a powerful diagnostic aid to help them identify cataracts and some types of ocular tumours in newborn babies.
Our client, a start-up called NeoCAM, required a low-cost handheld device suitable for use by clinicians as a diagnostic aid.
Cataract is the most common cause of avoidable child blindness in the world. Dr Louise Allen, CEO at NeoCAM, was frustrated by the number of babies with cataracts missed by the current screening process and the number of false positives.
What we did
42T developed the industrial design, user experience (UX) and system architecture, working closely with the client’s domain expert.
We then designed and produced the optics, electronics, casework, firmware and software for initial prototypes. These were used in a small-scale field trial in sub-Saharan Africa, to great success.
The NeoCAM device uses infra-red light which is invisible to the naked eye so the patient doesn’t notice anything, ideal for screening babies. The reflection from the back of the eye using infra-red is also bright and consistent which improves overall accuracy.
Significantly, the device eliminates the impact of eye pigmentation, which improves diagnosis in ethnic groups with darker eye pigmentation where false positives using the current method are common. This innovation, therefore, holds tremendous promise for better accuracy and diagnosis in these population groups, both in developing countries and in Europe.
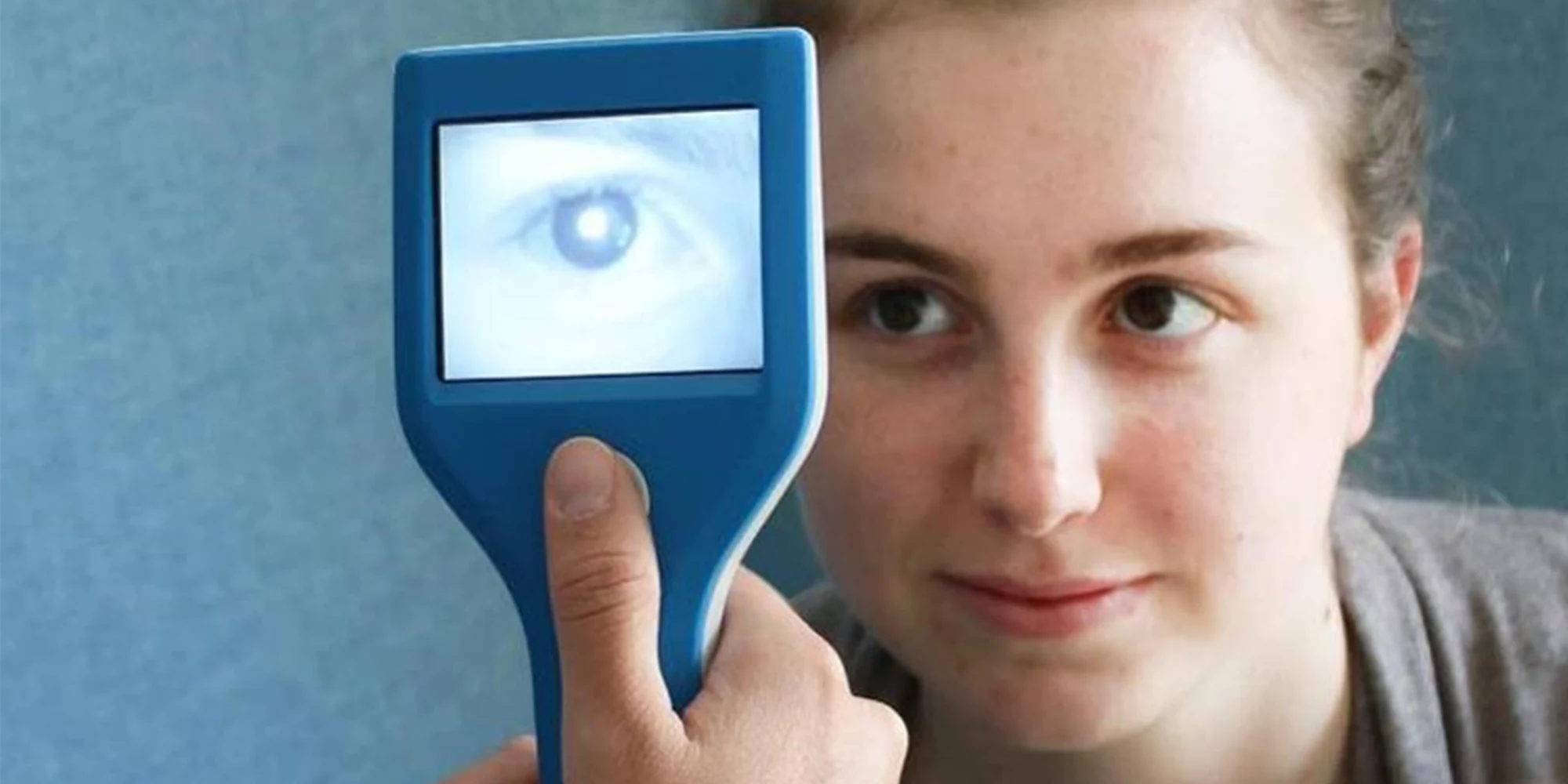

“Current screening methods are particularly less accurate for non-Caucasian babies. Early trials indicate that the IR reflex test is more accurate in these populations, and easy to use by inexperienced healthcare staff.”
Results for our client
Improved detection and earlier intervention will reduce babies’ risk of life-long visual impairment.
Results have so far shown better accuracy compared to the current method of newborn cataract screening – particularly for babies with darker skin tones.
42T was able to ably support an ophthalmic diagnostic device start-up, from concept through to clinical trials now underway in the UK.
Trials and updated software
A UK trial is now underway, aiming to screen 140,000 newborns and show that digital imaging is more accurate than current techniques. The data will later be used to train the software to aid diagnosis.
Dr Allen says, “As the trial progresses, we hope that the anonymised eye images collected will be used to develop and train the software used with the imaging device, allowing it to automatically alert the user when it recognises a potential problem. This means that eye screening for infants and children could potentially be made available in rural communities worldwide where there are fewer trained health professionals available.”
Sarah Knight, Head of Healthcare Technology at 42T, says, “We are extremely proud to have supported Dr Allen in driving the development of NeoCAM devices forward. At 42T we are passionate about expanding access to high-quality healthcare to all, so are excited that this device could mean more accurate screening in remote areas in the future.”
“This device is equally effective for babies with different skin tones, which is not the case for the traditional test. Technologies like this play a big part in furthering health equity.”
