42T, FIND, the global alliance for diagnostics, and Rutgers University (Rutgers) worked together to develop an innovative stool sample processing kit that has played a central role in informing the World Health Organization’s (WHO) recent policy update to improve the diagnosis of tuberculosis in children [ref 1].
In 2020, 1.1 million children fell ill with TB out of a total of around 10 million people worldwide. Although TB is treatable and preventable, it can be difficult to diagnose and treat in children [ref 2].
“Diagnosing paediatric TB is a major challenge requiring fresh thinking, because traditional samples for TB testing just aren’t accessible from young children. Stool is so easy to collect from almost anyone – so being able to use that to test for TB is a major step forward. FIND was pleased to work with 42T and other partners to help generate crucial data for the WHO review, and we warmly welcome the update to the guidelines.”
What we did
42T, Rutgers and FIND partnered to develop a simple, low-cost sample preparation kit.
42T’s design and development inputs for the SPK project included: working closely with Rutgers to help test out key device functions, to design prototype moulded devices, and to transfer the final designs into volume manufacture to produce initial quantities for use in clinical trials. This work was also supported by a grant from the US National Institute of Allergy and Infectious Diseases.

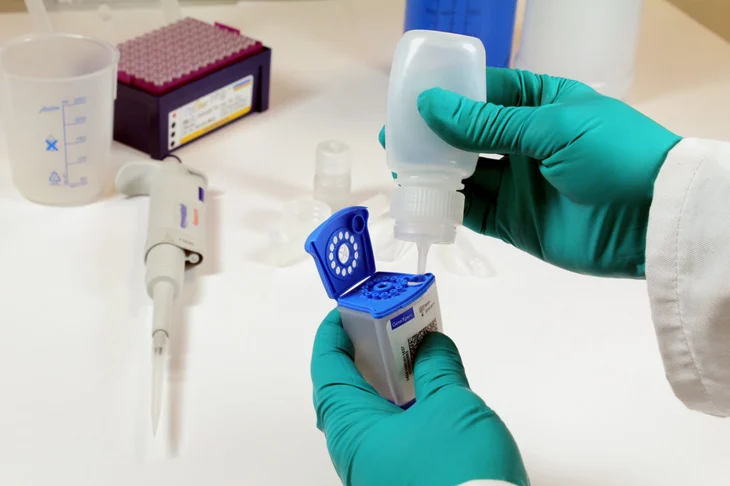
“Large-scale trials have shown the kit to be both effective and easy to use in a variety of settings. And we’re pleased that it has helped to inform the WHO in making its ground-breaking policy update on diagnosing childhood TB.”
Results for our client
42T and FIND have worked together on multiple long-term joint development projects over the last 10 years.
42T, Rutgers and FIND have successfully partnered to develop a simple, low-cost sample preparation kit, based on a design and process methodology first proposed by David Alland, director of the Public Health Research Institute at Rutgers University.
42T was specifically brought in for its creative approach in helping to solve complex and interrelated design problems that often occur when developing new diagnostics in readiness for private and public sector launch. The work has covered the development of diagnostics for TB, malaria and other diseases of the developing world.
References
1 Rapid communication on updated guidance on the management of tuberculosis in children and adolescents
https://www.who.int/news/item/26-08-2021-who-issues-rapid-communication-on-updated-guidance-for-the-management-of-tb-in-children-and-adolescents
2 WHO tuberculosis factsheet (updated 14 October 2021)
https://www.who.int/news-room/fact-sheets/detail/tuberculosis
3 On World Children’s Day, FIND welcomes improved access to childhood diagnosis of tuberculosis
https://www.finddx.org/newsroom/pr-20nov21/
4 Xpert MTB/RIF – rapid TB test – WHO publishes policy and guidance for implementers
https://www.who.int/news/item/18-05-2011-xpert-mtb-rif—rapid-tb-test—who-publishes-policy-and-guidance-for-implementers


